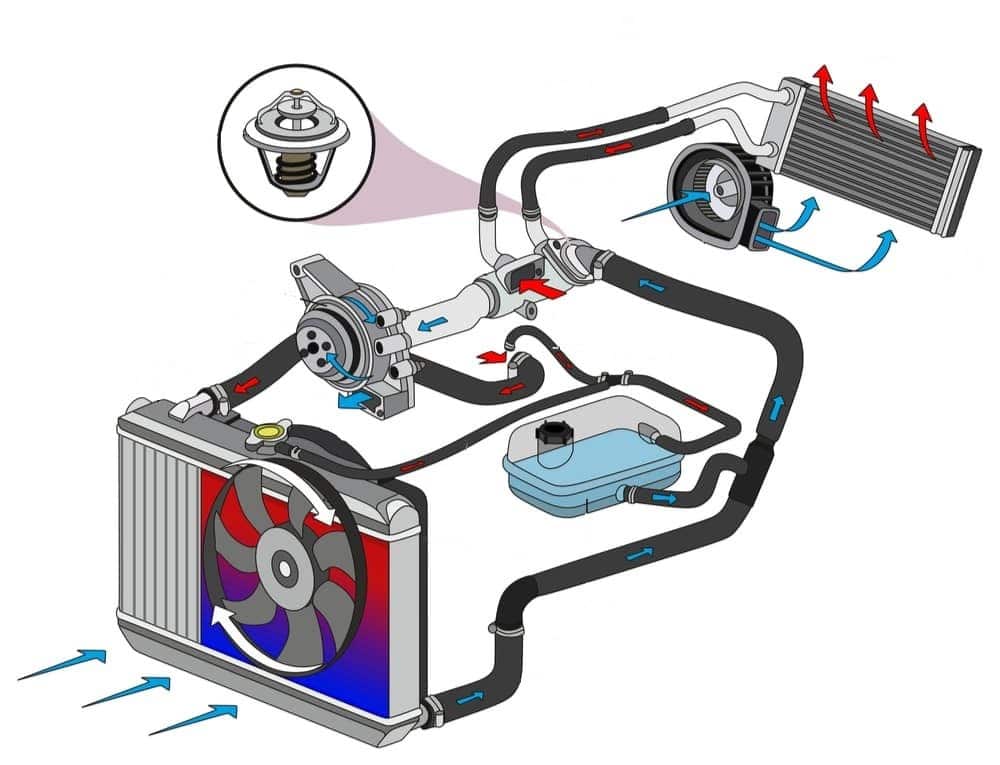
Antifreeze, as one of the chemical products, lowers the freezing point of fluids, protecting a system from the ill effects of ice formation in winter. It is also used in summer. Antifreeze can increase the boiling point of fluids to prevent devices and engines from boiling. Its main ingredient is ethylene glycol, which lowers the freezing point of water and raises its boiling point. It will prevent the radiator water from freezing, boiling, or evaporating. Antifreeze is produced in two different types: oil-based and alcohol-based.
Oil-based antifreeze has the strongest and highest resistance against the heat caused by the activity of engine parts. For this reason, it prevents damage to the washer or cylinder. The antifreeze has the highest level of protection for car engine parts. Also, in terms of cost, it has a reasonable price, and its lifespan is high and long, so its use is frequent.
Alcohol-based antifreeze consists of organic compounds and generally costs more than oil-based antifreeze. One of the most noticeable features of alcohol antifreeze is that it protects car engine parts against cold and heat. This antifreeze has a high resistance to freezing and is very suitable for the cold seasons of the year. It is precisely what an antifreeze should be. But if we want to compare this type of antifreeze with an oil-based one, it has a shorter lifespan, higher price, and less efficiency. Also, if you are not careful about pouring acidic antifreeze into the car radiator, spilling some of this antifreeze on the car body will destroy the color of the body.
Application
- Cooling the car engine
- Using for heating and air conditioning systems
- Preventing the pipes from bursting and causing damage
- Reducing the risk of sediment formation and corrosion
- Using it for solar water heating systems
- Removing ice from roads
Antifreeze properties
- Having a high boiling point
- Having a low freezing point
- Providing stability in a wide range of temperatures
- Having a high specific heat and thermal conductivity and improved heat transfer
- Having low viscosity
- Being chemically ineffective
- Reducing the need for thermostat, radiator, and water pump repairs
- Saving time and money in repair and maintenance
- Protecting against corrosion
- Being compatible with rubber and plastic
Types of antifreeze
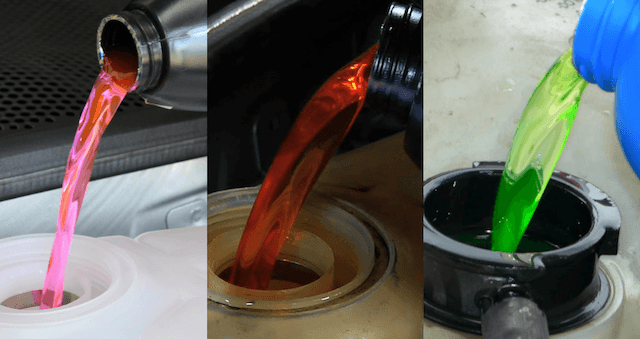
Organic Acid Technology (OAT) – organic acids – orange or red
- Inorganic Additive Technology (IAT) – silicate – green
- Hybrid OAT (HOAT) – silicates and organic acids – yellow
- Silicated HOAT (Si-OAT) – silicates and organic acids – purple
- Phosphate HOAT (P-HOAT) – phosphates and organic acids – blue, red, or pink
- Hybrid OAT (HOAT), without phosphate – without nitrite, amine and phosphate (NAP), turquoise
Liquid antifreeze is colored, but colors are added by request of manufacturers, usually choosing shades from green to blue, while organic antifreeze usually has shades from hot pink to orange.
However, although the antifreeze color is somewhat standardized, there is no strict requirement to specify it. On the other hand, many antifreezes are “universal,” meaning they meet different standards, so it’s best not to limit yourself by color.
The difference between antifreeze and coolant.
Antifreeze and coolant are different liquids. Antifreeze is a thick liquid mixed with distilled water before adding to the cooling system. Car manufacturers design their car cooling systems to work well with the adjusted coolant ratio. If the ratio of antifreeze to water is incorrect, the cooling system will not work well, and you may face problems such as deposit formation or overheating of the car engine.
Signs indicating the need for antifreeze
- Coolant leakage
- Engine overheating
- The steam coming off the hood
- Dirty or rusty radiator fluid
Antifreeze compounds
The antifreeze mainly consists of the following:
Base fluid: monoethylene glycol (MEG), propylene glycol, or glycerin.
- Additives: corrosion, cavitation, and sediment inhibitor, anti-foam, stabilizer, and buffer
The main difference between MEG and glycerin is that MEG gives antifreeze a more stable freezing point. Pure MEG causes the corrosion of ferrous and non-ferrous metals, so corrosion inhibitors are mandatory components of any antifreeze. There are about 5% additives and a maximum of 5% water, so about 90% of the antifreeze concentrate is made of ethylene glycol or the most common MEG.
Quality standards
A high-quality antifreeze must meet the quality requirements defined by Iran’s National Standard No. 338 and international military standards (ASTM, DEF, MIL, SAE) to determine the minimum quality and frequency of changing antifreeze.
Mixing different types of antifreeze
There are compatibility tables for mixing several types of antifreeze, but in addition to being c, they are not universal and often only apply to one manufacturer. It will impair the quality of the newer antifreeze and not improve the quality of the old (worse) antifreeze.
Because you can’t rely on the color, and doing chemical analyses is very costly, the usual drivers can’t reliably tell which antifreezes are compatible. Therefore, they should add the same antifreeze used already in the system or change it completely. In cases of emergency, even a low amount of distilled or non-mineral water can do the same. If higher amounts are required (for example, if a hose leaks), the best solution is to fix the flaw yourself or at a service center to prevent the engine from “boiling” or even losing more coolant.
Using water instead of coolant
Considering that the goal is to prevent freezing during the winter, water cannot be used as a coolant because it freezes at 0 degrees Celsius and can crack the cylinder block and head. Moreover, it simply evaporates in the summer due to the high temperature. In addition, water leads to the corrosion of metal parts and the formation of lime deposits, slowing down the fluid flow of the system and causing damage.
Mixing ratio of antifreeze and water
For preparing the engine coolant, it is necessary to add distilled or non-mineral water to the antifreeze, usually in a ratio of 50:50 or 60:40. it is necessary to add distilled or non-mineral water to the antifreeze, usually in a ratio of 50:50 or 60:40. The solutions of modern antifreeze types based on ethylene glycol with a concentration of 50% have a crystallization point between −35 and −40 °C, so they are widely used. Commonly used are 40-60% solutions of concentrated antifreeze that are usable between −28 °C and −50 °C. In hotter areas, a concentration of 30% is used because they only protect up to -18 °C, and lower concentrations do not protect against corrosion. Therefore, how much you mix antifreeze and water depends on the weather conditions. The best advice is to follow the instructions on the package because each manufacturer knows their product better.
The misconception is that higher concentrations provide about 70% better protection because they make the freezing point higher, and lower concentrations do not provide adequate protection against corrosion because they do not contain sufficient corrosion inhibitors. Despite that, concentrated antifreeze is not ideal because the base can be corrosive. In addition, concentrated antifreeze is more viscous and weaker in heat transfer, and even compared to its aqueous solutions, it has a significantly lower heat capacity.

